About Bruin Biosciences
Market Analysis for Interventional Radiology Products
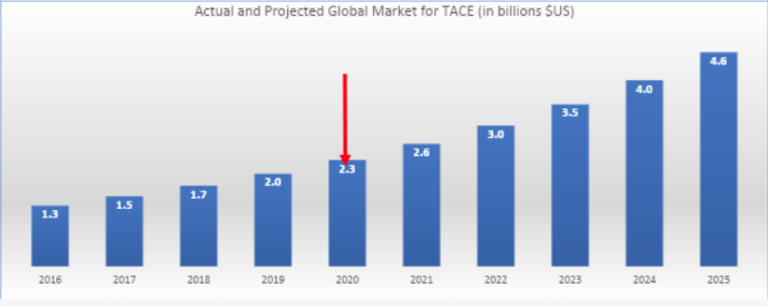
Market for DEBs
- Global market for embolics is expected to grow at 10-15% annually to approximately $4.6B by 2025; DEBs with approved drugs attached is very likely to significantly increase this market
- US and Europe are the largest markets for embolics, but Asia (especially China) is the fastest growing due to increasing incidence of HCC
- There are currently NO FDA approved drugs for use with DEB-TACE procedures in the US
Intellectual Property
PCT US1834744 filed May, 2018 details Bruin Bio formulations for:
- Approved HCC drugs: US Application No. 62/511,895
- General Platform: US Application No. 62/511,898
- Priority May 2017
- National stage filing done Nov 2019
Patent examiner’s rendered opinion stating claims are novel and inventive
Sorafenib became generic in January 12, 2020.
US patent on Regorafenib expires July 2024.
Current Proposed Development Path for Bruin Bio
Year 1
Year 2
Year 3

Proof-of-concept animal studies with controls; pharmacokinetics studies; safety study
Timeline: 6-9 months

Regulatory affairs related tasks for filing with FDA
Timeline: 6-12 months.

Phase 1 clinical study of 10-15 patients
Timeline: 12-18 months