David Imagawa, Nadine Abi-Jaoudeh, Jim Na, Fabio Tucci, Satheesh Ravula, Graham Beaton
Liver Cancer: A Pressing Unmet Medical Need
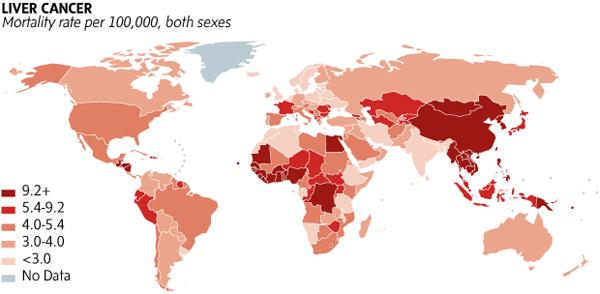
- Hepatocellular carcinoma (HCC) is the most deadly solid organ cancer worldwide. In 2020, 840,000 cases with >90% mortality rate
- In the United States, incidence of HCC has almost tripled since the 1980s and is the fastest rising cause of cancer related death. 38,000 cases in 2020.
- Incidence of liver cancer has increased each year since mid-1970s, a trend expected to continue through at least 2030. It is the ONLY common cancer which has been increasing in incidence*
ABOUT US
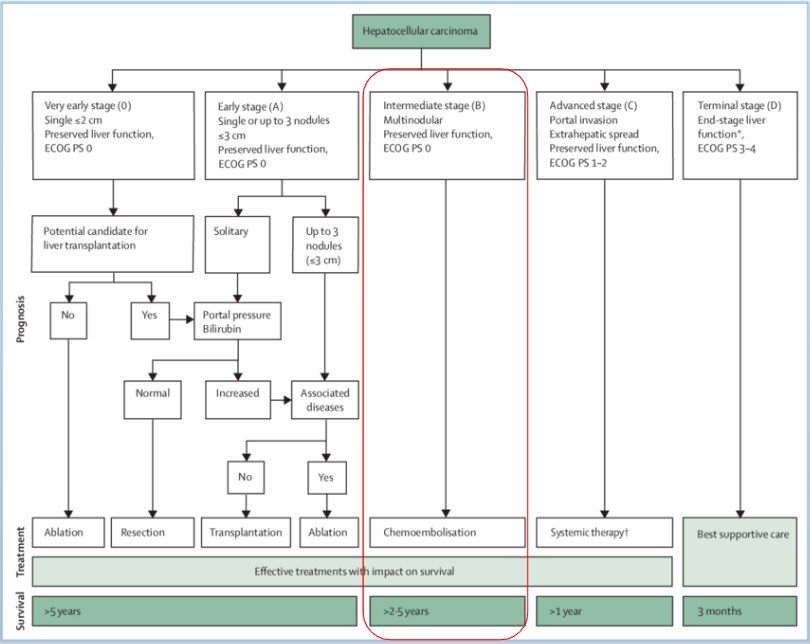
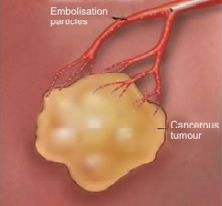
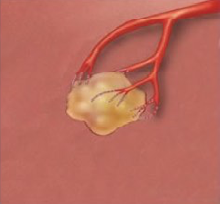
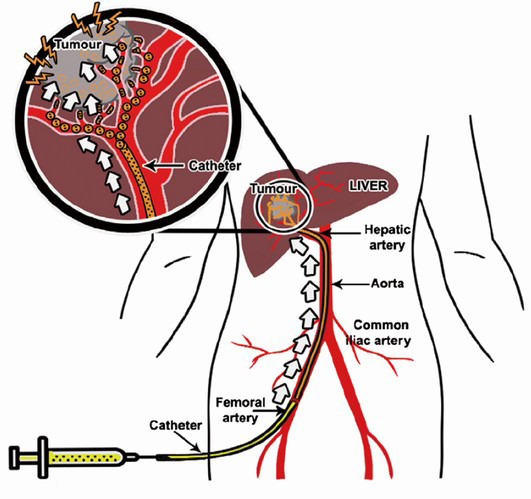
Trans arterial Chemoembolization (TACE)
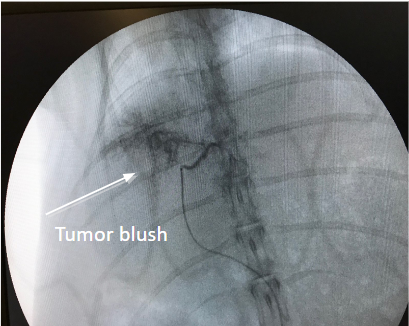
- Liver tumor receives blood mainly from the hepatic artery.
- Inserting embolization agents into hepatic artery to block off blood supply to the tumor can cause tumor necrosis.
- Drug that is attached to the beads can slowly release into the tumor, thereby minimizing systemic exposure, which leads to less side effects.
Current Limitations of TACE
- Chemotherapeutic agent must be water soluble
- Doxorubicin has minimal effects against HCC
Bruin Biosciences: Advancing Care for HCC
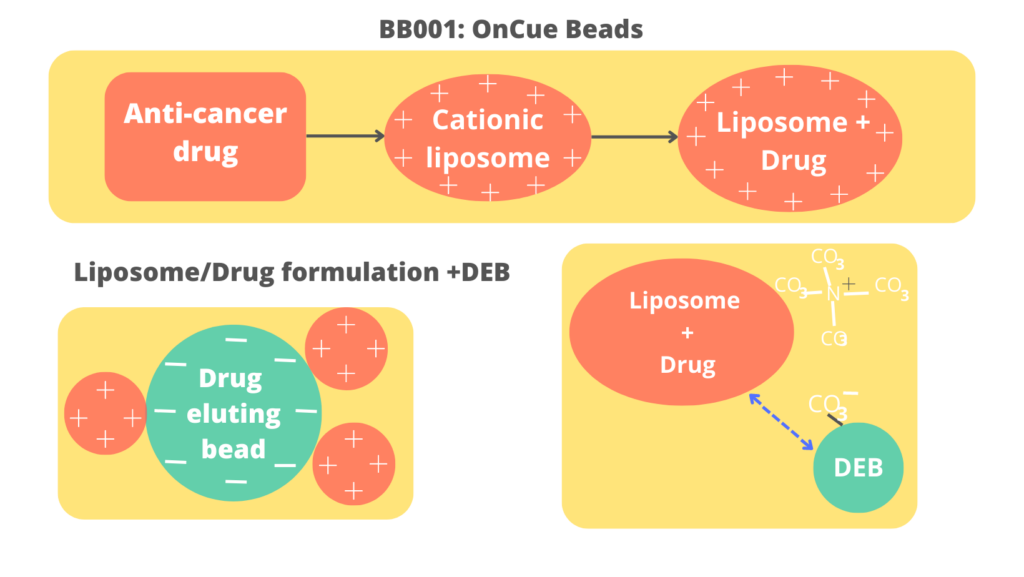
Bruin Bio developed a patented formulation to attached an FDA approved HCC drug, Nexavar (sorafenib) to a DEB to form our proof-of-concept product BB0001
- Bruin Bio developed a patented formulation to attached an FDA approved HCC drug, Nexavar (sorafenib) to a DEB to form our proof-of-concept product BB0001
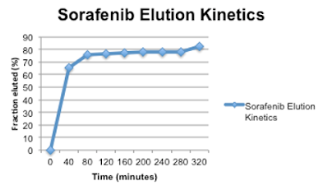
- BB0001 demonstrates suitable elution kinetics to afford clinically relevant drug concentrations
BB0002 is prepared with Stivarga (regorafenib), also FDA approved for HCC, with similar elution kinetics result
Liver Cancer Histology: 24 hour post treatment
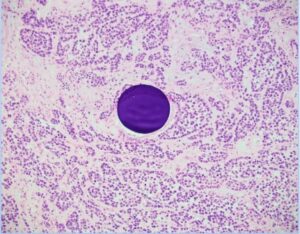
Control: Beads only
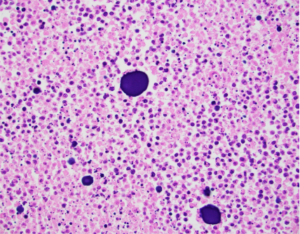
OnCue Beads + Sorafenib
Rabbit HCC Model: 5 days post Treatment
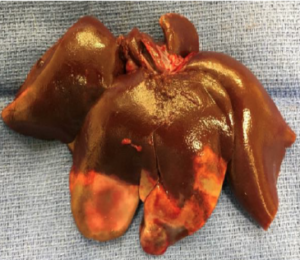
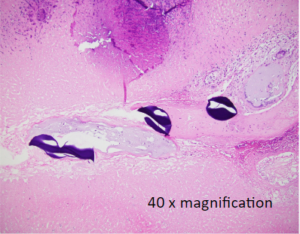
Local delivery: Tissue analysis of post-treatment tumor samples indicate complete tumor necrosis resulted from BB0001, indicating clinically efficacious concentrations of sorafenib was delivered to the tumor micro-environment
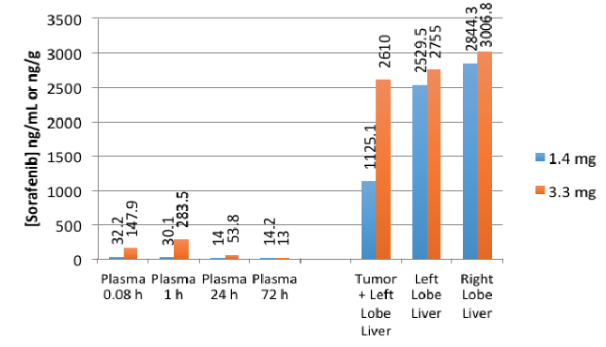
BB0001: Efficacious and Low Systemic Exposure
Less toxicity: Post treatment plasma and tissue concentrations of sorafenib were analyzed. Circulating plasma levels of sorafenib are 10-100x lower than tumor tissues drug concentration, and well below traditional therapeutic levels of drug following systemic chemotherapy
Liver Cancer in Dogs

HCC is most common tumor of the liver No breed pre disposition Age of onset usually over 10 years of age Treatment parallels human therapy
Treatment trial in conjunction with UC Davis School of Veterinary Medicine
Bruin Biosciences: TACE Platform
Clear advantage over current TACE procedures
Localized delivery of efficacious HCC drugs to the tumor should greatly improved TACE as a therapy
Less systemic side effects
Our animal studies demonstrate high drug concentration in the tumor with minimal systemic leakage
Prevent tumor re-growth
Combinations of anti-VEGF drugs (such as sorafenib) with DEB-TACE can be expected to prevent tumor re-growth
Extending the Bruin Biosciences Platform:
Perform clinical trial with BB0001 in HCC
Proof-of-concept for our first product to treat HCC patients
Pre-clinic studies for drug combinations
Formulate multiple cancer drugs to be delivered via TACE
Pursue other primary cancers
Renal cell carcinoma and osteosarcoma as primary indications
Pursue metastatic HCC
Colorectal, lung, stomach, pancreatic, esophagus, breast
Our Team
QUESTIONS?
If you have any questions, we’re here to answer